Cell Dynamics Lab
Morphogenesis,
from molecule to tissue
Mechanics of tissue morphogenesis
Molecular and cellular control of gastrulation in ascidians
With Kristin Sherrard, Ed Munro and Patrick Lemaire
We use simple model systems to understand tissue morphogenesis during embryonic development.
In our previous work, we focused on invagination, a simple mode of tissue deformation/folding, using gastrulation in the basal chordate Ciona intestinalis to understand the molecular underpinnings of this process.
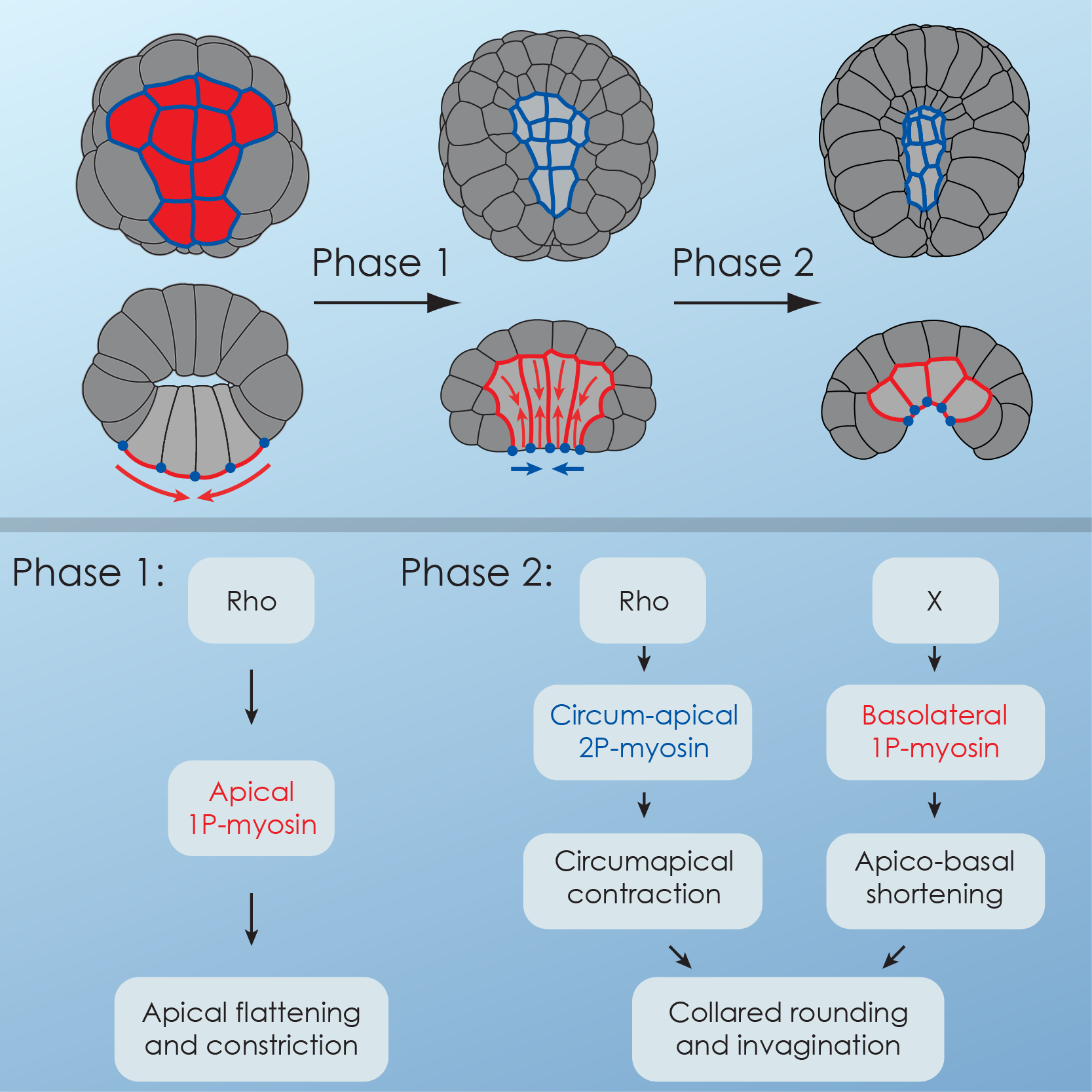
Combining 4D microscopy1-4, experimental manipulation of actomyosin contractility and computer simulation, we showed that sequential deployment of active myosin to different cell surfaces drove a 2-step sequence of endoderm cell shape changes during invagination5. During Step 1, apical accumulation of Myosin caused an apical constriction in the endodermal cells, but no invagination. During Step 2, the interplay between stable circumapical 2P-myosin and basolateral myosin in the endoderm, drove an apico-basal shortening of the cells around tightly maintained, preshrunk apices, resulting in an invagination of the endoderm (Fig. 1).
Neural tube closure in ascidians: zipping up a tissue
With Hidehiko Hashimoto, Kristin Sherrard and Ed Munro
Neurulation is one of the defining events of chordate morphogenesis, in which the neural tissue folds, elongates along the antero-posterior axis and meets at the midline in a process called zippering. While the first two steps of neurulation had been extensively studied, zippering remained poorly understood, and therefore represented a promising research avenue.
We initiated this project during a research apprenticeship at the Friday Harbor Labs. During this 10-weeks-long course, our group of students and instructors created quite a unique environment, stimulating scientifically and extremely dynamic. The initial dataset we collected during the research apprenticeship pointed to a very specific dynamics of zippering.
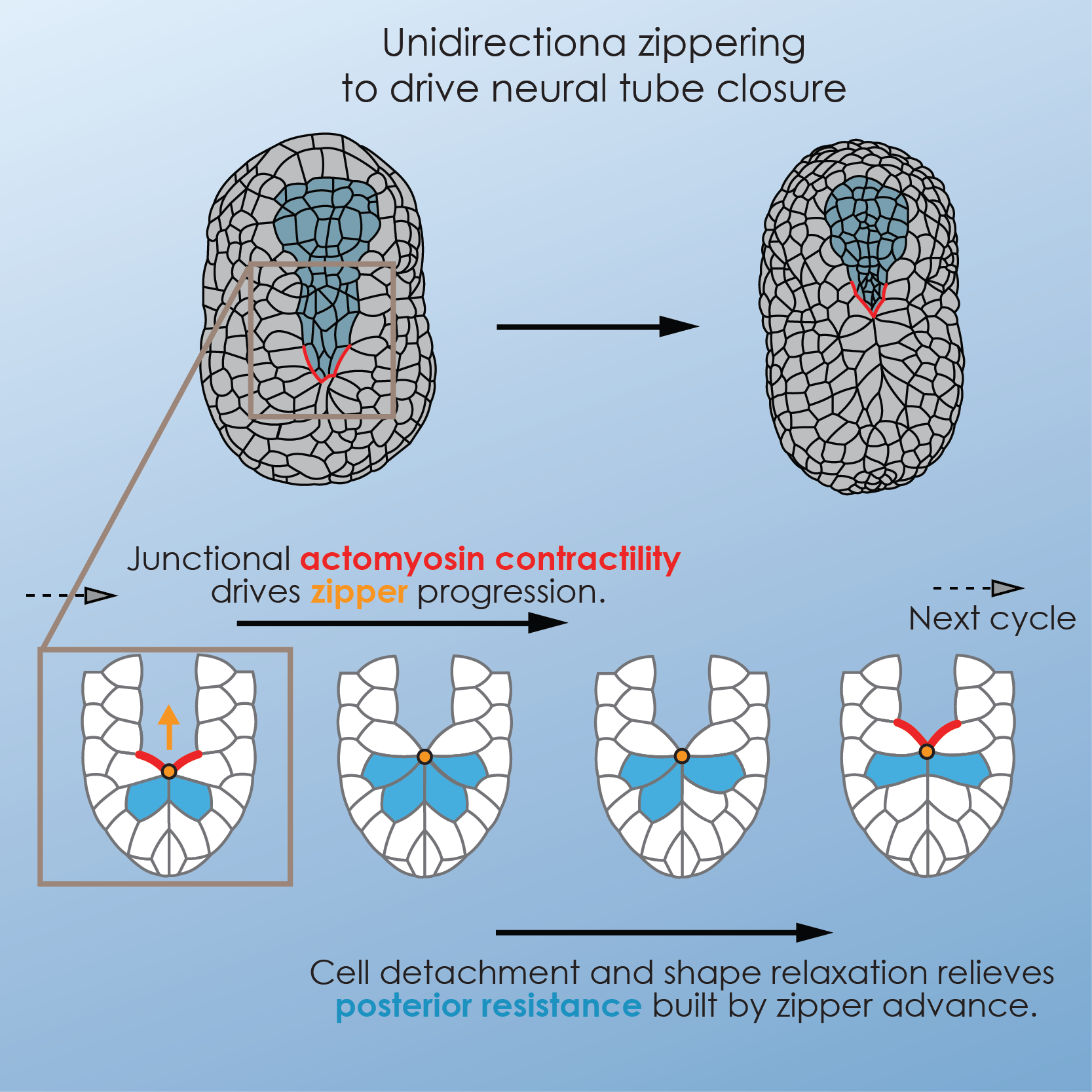
We further combined quantitative microscopy with physical and pharmacological manipulation and computer simulations to identify the cytomechanical basis for zippering and neural tube closure. Specifically, we demonstrated that sequential, Rho-Kinase-dependent, activation of myosin activity along the neural epidermal boundary ahead of the zipper caused shortening of the junction, while dynamic junction rearrangement of cells behind the zipper allowed cells stretched by the advancing zipper to detach and relax (Fig. 2). Using laser ablation, we measured tension along the junctions in the embryo and showed that Myosin activation resulted in local increase in tension ahead of the zipper. Finally, in combination with computer simulation, we demonstrated that (1) the proposed model recapitulated the hallmarks of the process, (2) zippering contributed actively to neural tube closure, (3) this mode of tissue deformation, based on local contractility and tension release, was very robust to global tissue deformations in comparison to a simple purse-string mechanism, and therefore could effectively concur with embryonic elongation, and (4) similar mechanisms were capable of driving zippering and neural tube closure in larger tissues, and therefore that zippering may be an essential component of neurulation in higher vertebrates, including humans6.
- A. Roure et al., PLoS ONE. 2, e916 (2007).
- F. B. Robin et al., Cold Spring Harb Protoc. 2011, 1244–1246 (2011).
- F. B. Robin et al., Cold Spring Harb Protoc. 2011, 1251–1261 (2011).
- F. B. Robin et al., Cold Spring Harb Protoc. 2011, 1247–1250 (2011).
- K. Sherrard, F. Robin, P. Lemaire, E. Munro, Curr. Biol. 20, 1499–1510 (2010).
- H. Hashimoto, F. B. Robin, K. M. Sherrard, E. M. Munro, Developmental Cell. 32, 241–255 (2015).
IBPS - Laboratoire de Biologie du Développement – ERL 1156
CNRS – Université Pierre et Marie Curie – Inserm
9, quai Saint-Bernard – Bât. C – 6ème Et. – Case 24 – 75252 Paris cedex 05 – France
Tel :+33 (0)1 44 27 42 41 – email : francois.robinATupmc.fr